the basic of thes chapther is understanding of Introduction to Oxidation Reduction (Redox) Reactions
Oxidation Reduction (Redox)
Oxidation and reduction, often abbreviated as redox, are chemical processes that involve the transfer of electrons between substances. These processes are fundamental to understanding many chemical reactions, including combustion, corrosion, and electrochemical reactions. Here’s an explanation of oxidation, reduction, and redox reactions:
- Oxidation:
- Oxidation refers to the loss of electrons by a substance, resulting in an increase in its oxidation state (or oxidation number).
- In oxidation, the substance that loses electrons is called the reducing agent or reductant because it causes the reduction of another substance.
- Examples of oxidation include the reaction of iron with oxygen to form rust (Fe2O3) and the reaction of magnesium with oxygen to form magnesium oxide (MgO).
- Reduction:
- Reduction refers to the gain of electrons by a substance, resulting in a decrease in its oxidation state (or oxidation number).
- In reduction, the substance that gains electrons is called the oxidizing agent or oxidant because it causes the oxidation of another substance.
- Examples of reduction include the reaction of copper ions (Cu^2+) with electrons to form copper metal (Cu) and the reaction of hydrogen ions (H^+) with electrons to form hydrogen gas (H2).
- Redox Reactions:
- Redox reactions are chemical reactions that involve both oxidation and reduction processes occurring simultaneously.
- In a redox reaction, one substance is oxidized (loses electrons) while another substance is reduced (gains electrons). The substance that is oxidized is the reducing agent, and the substance that is reduced is the oxidizing agent.
- Redox reactions are essential in many natural and industrial processes, including photosynthesis, respiration, and the generation of electricity in batteries.
The key concept in redox reactions is the transfer of electrons. Oxidation and reduction always occur together in a redox reaction; if one substance loses electrons (oxidation), another substance must gain those electrons (reduction) to conserve charge.
An easy way to remember oxidation and reduction is through the mnemonic “OIL RIG”:
- Oxidation Is Loss (of electrons)
- Reduction Is Gain (of electrons)
Overall, redox reactions play a crucial role in chemical transformations, energy production, and the functioning of biological systems. Understanding these processes is essential in chemistry, biology, environmental science, and many other fields of study.
Oxidation numbers
Determining oxidation numbers (also known as oxidation states) for elements in compounds or ions is important in understanding redox reactions and chemical bonding. Here are the general rules and guidelines to determine oxidation numbers:
- Free Elements:
- The oxidation number of an element in its elemental form (e.g., O2, H2, Na) is always zero.
- For monatomic ions (ions consisting of only one atom), the oxidation number is equal to the charge of the ion. For example, the oxidation number of Na^+ is +1, and the oxidation number of O^2- is -2.
- Oxygen (O):
- In most compounds, oxygen has an oxidation number of -2. Exceptions include peroxides (H2O2) where oxygen has an oxidation number of -1, and compounds with fluorine where oxygen can have positive oxidation numbers (OF2).
- Hydrogen (H):
- In most compounds, hydrogen has an oxidation number of +1 when bonded to nonmetals and -1 when bonded to metals (called hydrides).
- Group 1 Elements (Alkali Metals):
- Group 1 elements (Li, Na, K, ) always have an oxidation number of +1 in compounds.
- Group 2 Elements (Alkaline Earth Metals):
- Group 2 elements (Be, Mg, Ca, ) always have an oxidation number of +2 in compounds.
- Fluorine (F):
- Fluorine is always assigned an oxidation number of -1 in compounds. Other halogens (Cl, Br, I) typically have negative oxidation numbers as well, except when bonded to more electronegative elements.
- Sum of Oxidation Numbers:
- For neutral compounds, the sum of oxidation numbers of all elements must be zero.
- For polyatomic ions, the sum of oxidation numbers must equal the charge of the ion.
- Oxidation Number Changes:
- In redox reactions, oxidation numbers can change. The element undergoing oxidation increases its oxidation number, while the element undergoing reduction decreases its oxidation number.
Here are a few examples to illustrate how to determine oxidation numbers:
- In H2O (water), oxygen typically has an oxidation number of -2. Since there are two hydrogen atoms, each with an oxidation number of +1, the overall compound is neutral, and the oxidation number of hydrogen is +1.
- In SO4^2- (sulfate ion), oxygen typically has an oxidation number of -2. Since there are four oxygen atoms, the total oxidation number contributed by oxygen is -8. The overall charge of the ion is -2, so the oxidation number of sulfur must be +6 to balance the charge.
- In NaCl (sodium chloride), sodium (Group 1 element) always has an oxidation number of +1, and chlorine (halogen) always has an oxidation number of -1.
By following these rules and considering the overall charge of the compound or ion, you can determine the oxidation numbers of elements in a wide variety of compounds and ions.
what is Nernst equation?
The Nernst equation is a fundamental equation in electrochemistry that relates the standard cell potential (E°cell) of an electrochemical cell to the actual cell potential (Ecell) under non-standard conditions, such as when the concentrations of reactants and products are not equal to their standard states. It helps calculate the cell potential at any given concentration of reactants and products.
The general form of the Nernst equation for a galvanic cell (one that produces electrical energy from a spontaneous redox reaction) is given as:

The Nernst equation is particularly useful in calculating cell potentials at non-standard conditions, such as different concentrations of ions in solution. It allows for the determination of how changes in concentration affect the cell potential and the direction of spontaneous electron flow in the cell.
For example, in a galvanic cell where a copper electrode is in contact with a copper(II) ion solution and a zinc electrode is in contact with a zinc ion solution, the half-reactions are:
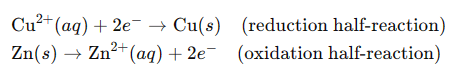
The standard cell potential for this cell can be determined from standard reduction potentials. The Nernst equation can then be used to calculate the cell potential under non-standard conditions, such as when the concentrations of copper(II) ions and zinc ions are not 1 M.
Overall, the Nernst equation provides a quantitative way to understand the relationship between cell potential, concentration, temperature, and the spontaneity of electrochemical reactions.
